Glucose
From Wikipedia, the free encyclopedia
| Glucose | |
|---|---|
 | |
 | |
| IUPAC name | |
| Other names | Dextrose |
| Identifiers | |
| Abbreviations | Glc |
| PubChem | |
| SMILES | |
| ChemSpider ID | |
| Properties | |
| Molecular formula | C6H12O6 |
| Molar mass | 180.156 g mol−1 |
| Density | 1.54 g cm−3 |
| Melting point | α-D-glucose: 146°C |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox references | |
Glucose (Glc), a monosaccharide (or simple sugar) also known as grape sugar, blood sugar, or corn sugar, is a very important carbohydrate in biology. The living cell uses it as a source of energy and metabolic intermediate. Glucose is one of the main products of photosynthesis and starts cellular respiration in both prokaryotes (bacteria and archaea) and eukaryotes (animals, plants, fungi, and protists).
The name glucose comes from the Greek word glykys (γλυκύς), meaning "sweet", plus the suffix "-ose" which denotes a sugar.
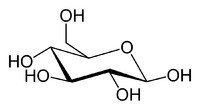
Two stereoisomers of the aldohexose sugars are known as glucose, only one of which (D-glucose) is biologically active. This form (D-glucose) is often referred to as dextrose monohydrate, or, especially in the food industry, simply dextrose (from dextrorotatory glucose[1]). This article deals with the D-form of glucose. The mirror-image of the molecule, L-glucose, cannot be metabolized by cells in the biochemical process known as glycolysis.
Contents[hide] |
[edit] Properties and energy content
The Gibbs free energy of formation of solid glucose is -909 kJ/mol and the enthalpy of formation is -1271.1 kJ/mol. The heat of combustion (with liquid water in the product) is about 2803 kJ/mol, or 3.72 kcal per gram. The ΔG (change of Gibbs free energy) for this combustion is about -2880 kJ/mol.
Upon heating, glucose, like any carbohydrate, will undergo caramelization, followed by pyrolysis (carbonization) yielding steam and a char consisting mostly of carbon. This reaction is exothermic, releasing about 0.237 kcal per gram.
[edit] Production
[edit] Natural
- Glucose is one of the products of photosynthesis in plants and some prokaryotes.
- In animals and fungi, glucose is the result of the breakdown of glycogen, a process known as glycogenolysis. In plants the breakdown substrate is starch.
- In animals, glucose is synthesized in the liver and kidneys from non-carbohydrate intermediates, such as pyruvate and glycerol, by a process known as gluconeogenesis.
[edit] Commercial
Glucose is produced commercially via the enzymatic hydrolysis of starch. Many crops can be used as the source of starch. Maize, rice, wheat, potato, cassava, arrowroot, and sago are all used in various parts of the world. In the United States, cornstarch (from maize) is used almost exclusively.
[edit] Function
Scientists can speculate on the reasons why glucose, and not another monosaccharide such as fructose (Fru), is so widely used in organisms. One reason might be that glucose has a lower tendency, as compared to other hexose sugars, to non-specifically react with the amino groups of proteins. This reaction (glycation) reduces or destroys the function of many enzymes. The low rate of glycation is due to glucose's preference for the less reactive cyclic isomer. Nevertheless, many of the long-term complications of diabetes (e.g., blindness, kidney failure, and peripheral neuropathy) are probably due to the glycation of proteins or lipids. In contrast, enzyme-regulated addition of glucose to proteins by glycosylation is often essential to their function.[2]
[edit] As an energy source
Glucose is an ubiquitous fuel in biology. It is used as an energy source in most organisms, from bacteria to humans. Use of glucose may be by either aerobic or anaerobic respiration (fermentation). Carbohydrates are the human body's key source of energy, through aerobic respiration, providing approximately 3.75 kilocalories (16 kilojoules) of food energy per gram.[3] Breakdown of carbohydrates (e.g. starch) yields mono- and disaccharides, most of which is glucose. Through glycolysis and later in the reactions of the Citric acid cycle (TCAC), glucose is oxidized to eventually form CO2 and water, yielding energy sources, mostly in the form of ATP. The insulin reaction, and other mechanisms, regulate the concentration of glucose in the blood. A high fasting blood sugar level is an indication of prediabetic and diabetic conditions.
Glucose is a primary source of energy for the brain, and hence its availability influences psychological processes. When glucose is low, psychological processes requiring mental effort (e.g., self-control, effortful decision-making) are impaired.[4][5][6][7]
[edit] Glucose in glycolysis
| ||||||||||||||||||||
| Compound C00031 at KEGG Pathway Database. Enzyme 2.7.1.1 at KEGG Pathway Database. Compound C00668 at KEGG Pathway Database. Reaction R01786 at KEGG Pathway Database. | ||||||||||||||||||||
Use of glucose as an energy source in cells is via aerobic or anaerobic respiration. Both of these start with the early steps of the glycolysis metabolic pathway. The first step of this is the phosphorylation of glucose by hexokinase to prepare it for later breakdown to provide energy.
The major reason for the immediate phosphorylation of glucose by a hexokinase is to prevent diffusion out of the cell. The phosphorylation adds a charged phosphate group so the glucose 6-phosphate cannot easily cross the cell membrane. Irreversible first steps of a metabolic pathway are common for regulatory purposes.
[edit] As a precursor
Glucose is critical in the production of proteins and in lipid metabolism. Also, in plants and most animals, it is a precursor for vitamin C (ascorbic acid) production. It is modified for use in these processes by the glycolysis pathway.
Glucose is used as a precursor for the synthesis of several important substances. Starch, cellulose, and glycogen ("animal starch") are common glucose polymers (polysaccharides). Lactose, the predominant sugar in milk, is a glucose-galactose disaccharide. In sucrose, another important disaccharide, glucose is joined to fructose. These synthesis processes also rely on the phosphorylation of glucose through the first step of glycolysis.
In industry it is a precursor to vitamine C in the Reichstein process.
[edit] Sources and absorption
All major dietary carbohydrates contain glucose, either as their only building block, as in starch and glycogen, or together with another monosaccharide, as in sucrose and lactose. In the lumen of the duodenum and small intestine, the glucose oligo- and polysaccharides are broken down to monosaccharides by the pancreatic and intestinal glycosidases. Other polysaccarhides cannot be processed by the human intestine and require assistance by intestinal flora if they are to be broken down; the most notable exceptions are sucrose (fructose-glucose) and lactose (galactose-glucose). Glucose is then transported across the apical membrane of the enterocytes by SLC5A1, and later across their basal membrane by SLC2A2.[8] Some of the glucose goes directly toward fueling brain cells, intestinal cells, and red blood cells, while the rest makes its way to liver, adipose tissue, and muscle cells, where it is absorbed (under the influence of insulin) and stored as glycogen. Liver cell glycogen is (when insulin is low or absent) converted to glucose and returned to the blood, muscle cell glycogen is not returned to the blood as the necessary enzymes are lacking. In fat cells, glucose is used to power reactions which, among other things, synthesize some fat types. Glycogen is the body's 'glucose energy' storage mechanism as it is much more 'space efficient' and less reactive than glucose itself.





No comments:
Post a Comment